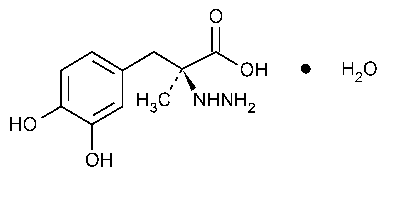
Carbidopa
Benzenepropanoic acid,a-hydrazino-3,4-dihydroxy-a-methyl-,monohydrate,(S)-.
(-)-L-a-Hydrazino-3,4-dihydroxy-a-methylhydrocinnamic acid monohydrate [38821-49-7].
Anhydrous 226.23 [28860-95-9].
»Carbidopa contains not less than 98.0percent and not more than 102.0percent of C10H14N2O4·H2O.
Packaging and storage—
Preserve in well-closed,light-resistant containers.
USP Reference standards á11ñ—
USP Carbidopa RS.USP Methyldopa RS.USP Carbidopa Related Compound A RS.
Identification—
A:
Infrared Absorption á197Mñ.
B:
Ultraviolet Absorption á197Uñ—
Solution:
40µg per mL.
Medium:
hydrochloric acid in methanol (1in 100).
Absorptivities at 282nm,calculated on the dried basis,do not differ by more than 3.0%.
Specific rotation á781Sñ:
between -21.0 and -23.5
and -23.5 ,calculated as the monohydrate.
,calculated as the monohydrate.
Test solution:
10mg per mL,in aluminum chloride solution (2in 3)that has been filtered and then adjusted with 0.25Nsodium hydroxide to a pHof 1.5.
Loss on drying—
Heat 1g,accurately weighed,in a suitable vacuum drying apparatus at 100 and at a pressure of not more than 5mm of mercury,to constant weight.Cool,and weigh:it loses not less than 6.9%and not more than 7.9%of its weight.
and at a pressure of not more than 5mm of mercury,to constant weight.Cool,and weigh:it loses not less than 6.9%and not more than 7.9%of its weight.
Residue on ignition á281ñ:
not more than 0.1%.
Heavy metals,Method IIá231ñ:
0.001%.
Limit of methyldopa and carbidopa related compound A—
Mobile phase,System suitability solution,Standard preparation,Assay preparation,and Chromatographic system—
Proceed as directed in the Assay.
Impurity standard preparation—
Dissolve accurately weighed quantities of USP Methyldopa RSand USP Carbidopa Related Compound A RSin Mobile phaseto obtain a solution having a known concentration of about 2.5µg of each per mL.
Procedure—
Separately inject equal volumes (about 20µL)of the Impurity standard preparationand the Assay preparationinto the chromatograph by means of a suitable sampling valve,and measure the peak responses.The relative retention times of methyldopa,carbidopa,and carbidopa related compound Aare about 0.8,1.0,and 1.8,respectively.Calculate the percentage of methyldopa in the portion of Carbidopa taken by the formula:
10(C/W)(rU/rS),
in which Cis the concentration,in µg per mL,of USP Methyldopa RSin the Impurity standard preparation;Wis the weight,in mg,of Carbidopa taken for the Assay preparation;and rUand rSare the peak responses for methyldopa obtained from the Assay and the Impurity standard preparation,respectively.The limit is 0.5%.Calculate the percentage of carbidopa related compound Ain the portion of Carbidopa taken by the formula:
10(C/W)(rU/rS),
in which Cis the concentration,in µg per mL,of USP Carbidopa Related Compound A RSin the Impurity standard preparation;Wis the weight,in mg,of carbidopa taken for the Assay preparation;and rUand rSare the peak responses for carbidopa related compound Aobtained from the Assay preparationand the Impurity standard preparation,respectively.The limit is 0.5%.
Organic volatile impurities,Method IVá467ñ:
meets the requirements.
Assay—
Mobile phase—
Prepare a filtered and degassed mixture of 0.05Mmonobasic sodium phosphate,adjusted with phosphoric acid to a pHof 2.7,and alcohol (95:5).Make adjustments if necessary (see System Suitabilityunder Chromatography á621ñ).
System suitability solution—
Prepare a solution in Mobile phasecontaining,in each mL,0.1mg of USP Carbidopa RSand 0.1mg of USP Methyldopa RS.
Standard preparation—
Dissolve an accurately weighed quantity of USP Carbidopa RSin Mobile phaseto obtain a solution having a known concentration of about 0.5mg per mL,using gentle heat and ultrasonification,if necessary,to aid dissolution.
Assay preparation—
Transfer about 50mg of Carbidopa,accurately weighed,to a 100-mLvolumetric flask,dilute with Mobile phaseto volume,and mix.
Chromatographic system(see Chromatography á621ñ)—
The liquid chromatograph is equipped with a 280-nm detector and a 3.9-mm ×30-cm column that contains packing L1.The flow rate is about 1mLper minute.Chromatograph the System suitability solution,and record the peak responses as directed for Procedure:the relative retention times are about 0.8for methyldopa and 1.0for carbidopa,and the resolution,R,between methyldopa and carbidopa is not less than 0.9.Chromatograph the Standard preparation,and record the peak responses as directed for Procedure:the relative standard deviation is not more than 1.5%.
Procedure—
Separately inject equal volumes (about 20µL)of the Standard preparationand the Assay preparationinto the chromatograph,record the chromatograms,and measure the responses for the major peaks.Calculate the quantity,in mg,of C10H14N2O4·H2Oin the portion of Carbidopa taken by the formula:
100C(rU/rS),
in which Cis the concentration,in mg per mL,of USP Carbidopa RS,as the monohydrate,in the Standard preparation,and rUand rSare the peak responses of the major peaks obtained from the Assay preparationand the Standard preparation,respectively.
Auxiliary Information—
Staff Liaison:Ravi Ravichandran,Ph.D.,Senior Scientist
Expert Committee:(PA3)Pharmaceutical Analysis 3
USP28–NF23Page 347
Pharmacopeial Forum:Volume No.30(3)Page 811
Phone Number:1-301-816-8330