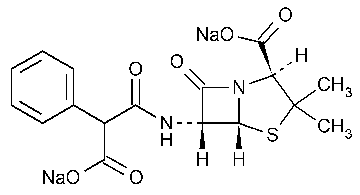
Carbenicillin Disodium
4-Thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid,6-[(carboxyphenylacetyl)amino]-3,3-dimethyl-7-oxo-,disodium salt,[2S-(2a,5a,6b)]-.
N-(2-Carboxy-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo.[3.2.0]-hept-6-yl)-2-phenylmalonamic acid disodium salt [4800-94-6].
»Carbenicillin Disodium has a potency equivalent to not less than 770µg of carbenicillin (C17H18N2O6S)per mg,calculated on the anhydrous basis.
Packaging and storage—
Preserve in tight containers.
Labeling—
Where it is intended for use in preparing injectable dosage forms,the label states that it is sterile or must be subjected to further processing during the preparation of injectable dosage forms.
Identification—
It responds to the tests for Sodium á191ñ.
pHá791ñ:
between 6.5and 8.0,in a solution containing 10mg of carbenicillin per mL.
Water,Method Iá921ñ:
not more than 6.0%.
Other requirements—
Where the label states that Carbenicillin Disodium is sterile,it meets the requirements for Sterilityand Bacterial endotoxinsunder Carbenicillin for Injection.Where the label states that Carbenicillin Disodium must be subjected to further processing during the preparation of injectable dosage forms,it meets the requirements for Bacterial endotoxinsunder Carbenicillin for Injection.
Assay—
Assay preparation—
Dissolve a suitable quantity of Carbenicillin Disodium,accurately weighed,in Buffer No.1,and dilute quantitatively with Buffer No.1to obtain a solution having a convenient concentration of carbenicillin.
Procedure—
Proceed as directed under Antibiotics—Microbial Assays á81ñ,using an accurately measured volume of Assay preparationdiluted quantitatively with Buffer No.1to yield a Test Dilutionhaving a concentration assumed to be equal to the median dose level of the Standard.
Auxiliary Information—
Staff Liaison:William W.Wright,Ph.D.,Scientific Fellow
Expert Committee:(PA7)Pharmaceutical Analysis 7
USP28–NF23Page 345
Phone Number:1-301-816-8335