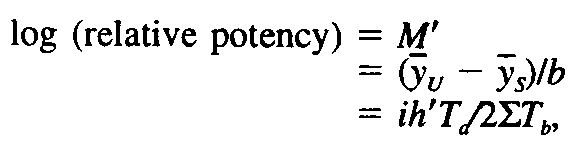á581ñVITAMIN D ASSAY
Chromatographic Method
The following pressurized liquid chromatographic procedure is provided for the determination of vitamin D,as cholecalciferol or as ergocalciferol,as an ingredient of Pharmacopeial multiple-vitamin preparations.
Throughout this assay,protect solutions containing,and derived from,the test specimen and the Reference Standard from the atmosphere and light,preferably by the use of a blanket of inert gas and low-actinic glassware.
USP Reference Standards á11ñ—
[NOTE—Use USP Ergocalciferol RS,or USP Cholecalciferol RS,for assaying pharmaceutical dosage forms that are labeled to contain vitamin Das ergocalciferol,or as cholecalciferol,respectively.]USP Cholecalciferol RS.USPD4,6-Cholestadienol RS.USP Ergocalciferol RS.USP Vitamin D Assay System Suitability RS.
Special Reagents and Solutions—
Ether—
Use ethyl ether.Use within 24hours after opening container.
Dehydrated Hexane—
Prepare a chromatographic column by packing a chromatographic tube,60cm ×8cm in diameter,with 500g of 50-to 250-µm chromatographic siliceous earth,activated by drying at 150 for 4hours (see Column Adsorption Chromatographyunder Chromatography á621ñ).Pass 500mLof hexanes through the column,and collect the eluate in a glass-stoppered flask.
for 4hours (see Column Adsorption Chromatographyunder Chromatography á621ñ).Pass 500mLof hexanes through the column,and collect the eluate in a glass-stoppered flask.
Butylated Hydroxytoluene Solution—
Dissolve a quantity of butylated hydroxytoluene in chromatographic hexane to obtain a solution containing 10mg per mL.
Aqueous Potassium Hydroxide Solution—
Dissolve 500g of potassium hydroxide in 500mLof freshly boiled water,mix,and cool.Prepare this solution fresh daily.
Alcoholic Potassium Hydroxide Solution—
Dissolve 3g of potassium hydroxide in 50mLof freshly boiled water,add 10mLof alcohol,dilute with freshly boiled water to 100mL,and mix.Prepare this solution fresh daily.
Sodium Ascorbate Solution—
Dissolve 3.5g of ascorbic acid in 20mLof 1Nsodium hydroxide.Prepare this solution fresh daily.
Sodium Sulfide Solution—
Dissolve 12g of sodium sulfide in 20mLof water,dilute with glycerin to 100mL,and mix.
Mobile Phase A—
Prepare a mixture of acetonitrile,methanol,and water (25:25:1).The amount of water and the flow rate may be varied to meet system suitability requirements.
Mobile Phase B—
Prepare a 3in 1000mixture of n-amyl alcohol in Dehydrated Hexane.The ratio of components and the flow rate may be varied to meet system suitability requirements.
Internal Standard Solution—
Transfer 15mg of USPD4,6-Cholestadienol RS,accurately weighed,to a 200-mLvolumetric flask,add a 1in 10mixture of toluene and Mobile Phase Bto volume,and mix.
Standard Preparation—
Transfer about 25mg of USP Ergocalciferol RSor Cholecalciferol RS,accurately weighed,to a 50-mLvolumetric flask,dissolve without heat in toluene,add toluene to volume,and mix.Pipet 10mLof this stock solution into a 100-mLvolumetric flask,dilute with toluene to volume,and mix.Prepare stock solution fresh daily.
Assay Preparation—
For oily solutions—
Accurately weigh a portion of the specimen to be assayed,preferably more than 0.5g and equivalent to about 125µg of cholecalciferol or ergocalciferol (5000USP Units).Add 1mLof Sodium Ascorbate Solution,25mLof alcohol,and 2mLof Aqueous Potassium Hydroxide Solution,and mix.
For capsules or tablets—
Reflux not less than 10capsules or tablets with a mixture of 10mLof Sodium Ascorbate Solutionand 2drops of Sodium Sulfide Solutionon a steam bath for 10minutes,crush any remaining solids with a blunt glass rod,and continue heating for 5minutes.Cool,add 25mLof alcohol and 3mLof Aqueous Potassium Hydroxide Solution,and mix.
For dry preparations and aqueous dispersions—
Accurately weigh a portion of the specimen to be assayed,preferably more than 0.5g and equivalent to about 125µg of cholecalciferol or ergocalciferol (5000USP Units).Add,in small quantities and with gentle swirling,25mLof alcohol,5mLof Sodium Ascorbate Solution,and 3mLof Aqueous Potassium Hydroxide Solution.
SAPONIFICATION AND EXTRACTION—
Reflux the mixture prepared from the specimen to be assayed on a steam bath for 30minutes.Cool rapidly under running water,and transfer the saponified mixture to a conical separator,rinsing the saponification flask with two 15-mLportions of water,10mLof alcohol,and two 50-mLportions of ether.Shake the combined saponified mixture and rinsings vigorously for 30seconds,and allow to stand until both layers are clear.Transfer the aqueous phase to a second conical separator,add a mixture of 10mLof alcohol and 50mLof solvent hexane,and shake vigorously.Allow to separate,transfer the aqueous phase to a third conical separator,and transfer the hexane phase to the first separator,rinsing the second separator with two 10-mLportions of solvent hexane,adding the rinsings to the first separator.Shake the aqueous phase in the third separator with 50mLof solvent hexane,and add the hexane phase to the first separator.Wash the combined ether-hexane extracts by shaking vigorously with three 50-mLportions of Alcoholic Potassium Hydroxide Solution,and wash with 50-mLportions of water vigorously until the last washing is neutral to phenolphthalein.Drain any remaining drops of water from the combined ether-hexane extracts,add 2sheets of 9-cm filter paper,in strips,to the separator,and shake.Transfer the washed ether-hexane extracts to a round-bottom flask,rinsing the separator and paper with solvent hexane.Combine the hexane rinsings with the ether-hexane extracts,add 5.0mLof Internal Standard Solutionand 100µLof Butylated Hydroxytoluene Solution,and mix.Evaporate to dryness in vacuum by swirling in a water bath maintained at a temperature not higher than 40 .Cool under running water,and introduce nitrogen sufficient to restore atmospheric pressure.Without delay,dissolve the residue in 5.0mLof a mixture of equal volumes of acetonitrile and methanol,or in a measured portion of the acetonitrile-methanol mixture until the concentration of vitamin Dis about 25µg per mL,to obtain the Assay Preparation.
.Cool under running water,and introduce nitrogen sufficient to restore atmospheric pressure.Without delay,dissolve the residue in 5.0mLof a mixture of equal volumes of acetonitrile and methanol,or in a measured portion of the acetonitrile-methanol mixture until the concentration of vitamin Dis about 25µg per mL,to obtain the Assay Preparation.
Chromatographic System—
Use a chromatograph,operated at room temperature,fitted with an UVdetector that monitors absorption at 254nm,a 30-cm ×4.6-mm stainless steel cleanup column packed with column packing L7and using Mobile Phase A,and a 25-cm ×4.6-mm stainless steel analytical column packed with column packing L3and using Mobile Phase B.
Cleanup Column System Suitability Test—
Pipet 5mLof the Standard Preparationinto a round-bottom flask fitted with a reflux condenser,and add 2or 3crystals of butylated hydroxytoluene.Displace the air with nitrogen,and heat in a water bath maintained at a temperature of 90 in subdued light under an atmosphere of nitrogen for 45minutes,to obtain a solution containing vitamin Dand pre-vitamin D.Cool,add 10.0mLof Internal Standard Solution,mix,and evaporate in vacuum to dryness by swirling in a water bath maintained at a temperature not higher than 40
in subdued light under an atmosphere of nitrogen for 45minutes,to obtain a solution containing vitamin Dand pre-vitamin D.Cool,add 10.0mLof Internal Standard Solution,mix,and evaporate in vacuum to dryness by swirling in a water bath maintained at a temperature not higher than 40 .Cool under running water,and introduce nitrogen sufficient to restore atmospheric pressure.Without delay,dissolve the residue in 10.0mLof a mixture of equal volumes of acetonitrile and methanol,and mix.Inject 500µLof this solution into the cleanup column,and record the chromatogram as directed under Procedure.The chromatogram exhibits a peak exhibiting a retention time between 5and 9minutes,corresponding to the separation under a single peak of the mixture of vitamin D,pre-vitamin D,and D4,6-cholestadienol from other substances.Adjust the water content or other operating parameters,if necessary (see Mobile Phase A).
.Cool under running water,and introduce nitrogen sufficient to restore atmospheric pressure.Without delay,dissolve the residue in 10.0mLof a mixture of equal volumes of acetonitrile and methanol,and mix.Inject 500µLof this solution into the cleanup column,and record the chromatogram as directed under Procedure.The chromatogram exhibits a peak exhibiting a retention time between 5and 9minutes,corresponding to the separation under a single peak of the mixture of vitamin D,pre-vitamin D,and D4,6-cholestadienol from other substances.Adjust the water content or other operating parameters,if necessary (see Mobile Phase A).
Analytical Column System Suitability Test—
Transfer about 100mg of USP Vitamin D Assay System Suitability RSto a 100-mLvolumetric flask,add a 1in 20mixture of toluene and Mobile Phase Bto volume,and mix.Heat a portion of this solution,under reflux,at 90 for 45minutes,and cool.Chromatograph five injections of the resulting solution,and measure the peak responses as directed for Procedure:the resolution,R,between trans-cholecalciferol and pre-cholecalciferol is not less than 1.0.and the relative standard deviation for the cholecalciferol peak response does not exceed 2.0%.[NOTE—Chromatograms obtained as directed for this test exhibit relative retention times of approximately 0.4for pre-cholecalciferol,0.5for trans-cholecalciferol,and 1.0for cholecalciferol.]
for 45minutes,and cool.Chromatograph five injections of the resulting solution,and measure the peak responses as directed for Procedure:the resolution,R,between trans-cholecalciferol and pre-cholecalciferol is not less than 1.0.and the relative standard deviation for the cholecalciferol peak response does not exceed 2.0%.[NOTE—Chromatograms obtained as directed for this test exhibit relative retention times of approximately 0.4for pre-cholecalciferol,0.5for trans-cholecalciferol,and 1.0for cholecalciferol.]
Calibration—
Vitamin D Response Factor—
Transfer 4.0mLof the Standard Preparationand 10.0mLof Internal Standard Solutionto a 100-mLvolumetric flask,dilute with Mobile Phase Bto volume,and mix to obtain the Working Standard Preparation.Store this Working Standard Preparationat a temperature not above 0 ,retaining the unused portion for the Procedure.Inject 200µLof the Working Standard Preparationinto the analytical column,and measure the peak responses for vitamin Dand for D4,6-cholestadienol.The relative retention time of D4,6-cholestadienol is about 1.3.Calculate the response factor,FD,by the formula:
,retaining the unused portion for the Procedure.Inject 200µLof the Working Standard Preparationinto the analytical column,and measure the peak responses for vitamin Dand for D4,6-cholestadienol.The relative retention time of D4,6-cholestadienol is about 1.3.Calculate the response factor,FD,by the formula:
CS/(RSCR),
in which CSand CRare the concentrations,in µg per mL,of vitamin Dand D4,6-cholestadienol,respectively,in the Working Standard Preparation,and Rsis the ratio of the peak response of vitamin Dto that of D4,6-cholestadienol.
Pre-Vitamin D Response Factor—
Pipet 4mLof the Standard Preparationinto a round-bottom flask fitted with a reflux condenser,and add 2or 3crystals of butylated hydroxytoluene.Displace the air with nitrogen,and heat in a water bath maintained at a temperature of 90 in subdued light under a nitrogen atmosphere for 45minutes,to obtain a solution containing vitamin Dand pre-vitamin D.Cool,transfer with the aid of several portions of Mobile Phase Bto a 100-mLvolumetric flask containing 10.0mLof Internal Standard Solution,dilute with Mobile Phase Bto volume,and mix to obtain the Working Mixture.Inject 200µLof this Working Mixtureinto the analytical column,and measure the peak responses for vitamin D,pre-vitamin D,and D4,6-cholestadienol.Calculate the concentration,C¢s,in µg per mL,of vitamin Din the (heated)Working Mixtureby the formula:
in subdued light under a nitrogen atmosphere for 45minutes,to obtain a solution containing vitamin Dand pre-vitamin D.Cool,transfer with the aid of several portions of Mobile Phase Bto a 100-mLvolumetric flask containing 10.0mLof Internal Standard Solution,dilute with Mobile Phase Bto volume,and mix to obtain the Working Mixture.Inject 200µLof this Working Mixtureinto the analytical column,and measure the peak responses for vitamin D,pre-vitamin D,and D4,6-cholestadienol.Calculate the concentration,C¢s,in µg per mL,of vitamin Din the (heated)Working Mixtureby the formula:
FDCRR¢S,
in which CRis the concentration,in µg per mL,of D4,6-cholestadienol,and R¢Sis the ratio of the peak response for vitamin Dto that forD4,6-cholestadienol.Calculate the concentration,C¢PRE,in µg per mL,of pre-vitamin D,in the Working Mixtureby the formula:
C¢PRE=CS–C¢S.
Calculate the response factor,FPRE,for pre-vitamin Dby the formula:
(FDR¢SC¢PRE)/(R¢PREC¢S),
in which R¢PREis the ratio of the peak response of pre-vitamin Dto that of D4,6-cholestadienol.[NOTE—Value of FPREdetermined in duplicate,on different days,can be used during the whole procedure.]
Procedure—
Inject 500µLof the Assay Preparationinto the cleanup column,and collect the fraction representing 0.7to 1.3relative to the retention time of the mixed vitamin Dpeak (see Cleanup Column System Suitability Test)in a round-bottom flask.Add 50µLof Butylated Hydroxytoluene Solution,mix,and evaporate in vacuum to dryness by swirling in a water bath maintained at a temperature not higher than 40 .Cool under running water,and introduce nitrogen sufficient to restore atmospheric pressure.Without delay,dissolve the residue in 5.0mLof a 1in 20mixture of toluene and Mobile Phase B,and mix.Inject 200µLof this solution into the analytical column,and measure the peak responses for vitamin D,pre-vitamin D,and D4,6-cholestadienol.Calculate the concentration,in µg per mL,of cholecalciferol (C27H44O)or ergocalciferol (C28H44O)in the Assay Preparationby the formula:
.Cool under running water,and introduce nitrogen sufficient to restore atmospheric pressure.Without delay,dissolve the residue in 5.0mLof a 1in 20mixture of toluene and Mobile Phase B,and mix.Inject 200µLof this solution into the analytical column,and measure the peak responses for vitamin D,pre-vitamin D,and D4,6-cholestadienol.Calculate the concentration,in µg per mL,of cholecalciferol (C27H44O)or ergocalciferol (C28H44O)in the Assay Preparationby the formula:
(R¢¢DFD+R¢¢PREFPRE)C¢¢R,
in which R¢¢Dis the ratio of the peak response of vitamin Dto that of D4,6-cholestadienol;R¢¢PREis the ratio of the peak response of pre-vitamin Dto that of D4,6-cholestadienol;and C¢¢Ris the concentration,in µg per mL,of D4,6-cholestadienol in the Assay Preparation.
Chemical Method
The following procedure is provided for the determination of vitamin Das an ingredient of Pharmacopeial preparations.
Complete the assay promptly,and exercise care throughout the procedure to keep to a minimum the exposure to air and to actinic light,preferably by the use of a blanket of inert gas and low-actinic glassware.
USP Reference Standards á11ñ—
[NOTE—Use USP Ergocalciferol RS,or USP Cholecalciferol RS,for assaying pharmaceutical dosage forms that are labeled to contain vitamin Das ergocalciferol,or as cholecalciferol,respectively.]USP Cholecalciferol RS.USP Ergocalciferol RS.
Special Reagents and Solutions—
Chromatographic Fuller's Earth—
Use chromatographic Fuller's earth having a water content corresponding to between 8.5%and 9.0%of loss on drying.
Solvent Hexane—
Use solvent hexane (see under Reagents,Indicators,and Solutions),redistilling if necessary so that it meets the following additional specification:
SPECTRAL PURITY—Measure in a 1-cm cell at 300nm,with a suitable spectrophotometer,against air as the blank:the absorbance is not more than 0.070.
Ethylene Dichloride—
Purify by passage through a column of granular (20to 200mesh)silica gel.
Potassium Hydroxide Solution—
Dissolve 500g of potassium hydroxide in water to make 1000mL.
Butylated Hydroxytoluene Solution—
Dissolve 10mg of butylated hydroxytoluene in 100mLof alcohol.Prepare this solution fresh daily.
Ether—
Use freshly distilled ether,discarding the first and last 10%portions of the distillate.
Color Reagent—
Prepare two stock solutions as follows.
SOLUTION A—Empty,without weighing,the entire contents of a previously unopened 113-g bottle of dry,crystalline antimony trichloride into a flask containing about 400mLof Ethylene Dichloride.Add about 2g of anhydrous alumina,mix,and pass through filter paper into a clear-glass,glass-stoppered container calibrated at 500mL.Dilute with Ethylene Dichlorideto 500mL,and mix:the absorbance of the solution,measured in a 20-mm cell at 500nm,with a suitable spectrophotometer,against Ethylene Dichloride,does not exceed 0.070.
SOLUTION B—Mix,under a hood,100mLof acetyl chloride and 400mLof Ethylene Dichloride.
Mix 45mLof Solution Aand 5mLof Solution Bto obtain the Color Reagent.Store in a tight container,and use within 7days,but discard any reagent in which a color develops.
Chromatographic Tubes—
First Column—
Arrange for descending column chromatography a tube of 2.5-cm (inside)diameter,about 25cm long,and constricted to 8-mm diameter for a distance of 5cm at the lower end,by inserting at the point of constriction a coarse-porosity,sintered-glass disk or a small plug of glass wool.The constricted portion may be fitted with an inert,plastic stopcock.
Second Column—
Select a tube that is made up of three sections:(1)a flared top section,18mm in (inside)diameter and approximately 14cm long,(2)a middle section,6mm in (inside)diameter and approximately 25cm long,and (3)a tapered,constricted lower exit tube approximately 5cm long.Insert a small plug of glass wool in the upper 1-cm portion of the constricted section.
Chromatographic Columns—
First Column—
To about 125mLof isooctane contained in a screw-capped,wide-mouth bottle add 25g of chromatographic siliceous earth,and shake until a slurry is formed.Add,dropwise and with vigorous mixing,10mLof polyethylene glycol 600.Replace the bottle cover,and shake vigorously for 2minutes.Pour about half of the resulting slurry into the chromatographic tube,and allow it to settle by gravity.Then apply gentle suction,and add the remainder of the slurry in small portions,packing each portion with a 20-mm disk plunger.When a solid surface has formed,remove the vacuum,and add about 2mLof isooctane.
Second Column—
Pack the midsection of the tube with 3g of moderately coarse Chromatographic Fuller's Earthwith the aid of gentle suction (about 125mm of mercury).
Standard Preparation—
Dissolve about 25mg of Reference Standard,accurately weighed,in isooctane to give a known concentration of about 250µg per mL.Store in a refrigerator.
On the day of assay,pipet 1mLof this solution into a 50-mLvolumetric flask,remove the solvent with a stream of nitrogen,and dissolve the residue in and dilute with Ethylene Dichlorideto volume,and mix.
Sample Preparation—
Accurately weigh or measure a portion of the sample to be assayed,equivalent to not less than 125µg but preferably about 250µg of ergocalciferol (10,000USP Units).If little or no vitamin Ais present in the sample,add about 1.5mg (the equivalent of 3000USP Units)of vitamin Aacetate to provide the needed pilot bands in the subsequent chromatography.
For capsules or tablets,reflux not fewer than 10of them in 10mLof water on a steam bath for about 10minutes,crush the remaining solid with a blunt glass rod,and warm for 5minutes longer.
Add a volume of Potassium Hydroxide Solutionrepresenting 2.5mLfor each g of the total weight of the sample,but not less than a total of 3.0mL.Add 10mLof Butylated Hydroxytoluene Solutionand 20mLof alcohol.Reflux vigorously on a steam bath for 30minutes.Cool,and transfer the saponified mixture to a conical separator,rinsing the saponification flask with three 10-mLportions of water and three 50-mLportions of Ether,adding each rinse to the separator.Add about 4g of sodium sulfate decahydrate to the separator,and extract by shaking for 2minutes.If an emulsion forms,extract with three 25-mLportions of Ether.Combine the ether extracts,if necessary,and wash by swirling gently with 50mLof water.Repeat the washing more vigorously with additional 50-mLportions of water until the last portion shows no pink color on the addition of phenolphthalein TS.Transfer the washed ether extract to a 250-mLvolumetric flask,dilute with Etherto volume,and mix.Transfer the entire sample or an accurately measured aliquot containing about 250µg to a tall-form,400-mLbeaker containing about 5g of anhydrous sodium sulfate.Stir for 2minutes,then decant the solution into a second 400-mLbeaker.Rinse the sodium sulfate with three 25-mLportions of Ether,adding each rinse to the main portion.Reduce the total volume to about 30mLby evaporation on a steam bath,and transfer the concentrate to a small,round-bottom evaporation flask.Rinse the beaker with three 10-mLportions of Ether,adding the rinsings to the flask.With the aid of vacuum in a water bath at a temperature not exceeding 40 ,or with a stream of nitrogen at room temperature,remove the remaining solvent completely.Dissolve the residue in a small amount of Solvent Hexane,transfer to a 10-mLvolumetric flask,dilute with Solvent Hexaneto volume,and mix to obtain the Sample Preparation.
,or with a stream of nitrogen at room temperature,remove the remaining solvent completely.Dissolve the residue in a small amount of Solvent Hexane,transfer to a 10-mLvolumetric flask,dilute with Solvent Hexaneto volume,and mix to obtain the Sample Preparation.
Procedure—
First Column Chromatography—
Just as the 2mLof isooctane disappears into the surface of the prepared First Column,pipet 2mLof the Sample Preparationonto the column.As the meniscus of the Sample Preparationreaches the column surface,add the first of three 2-mLportions of Solvent Hexane,adding each succeeding portion as the preceding portion disappears into the column.Continue adding Solvent Hexanein portions of 5to 10mLuntil 100mLhas been added.If necessary,adjust the flow rate to between 3and 6mLper minute,by application of gentle pressure at the top of the chromatographic tube.
Discard the first 20mLof effluent,and collect the remainder.Examine the column under UVlight at intervals during the chromatography,and stop the flow when the front of the fluorescent band representing vitamin Ais about 5mm from the bottom of the column.(The UVlamp should provide weakradiation in the 300-nm region.It is frequently necessary to use a narrow aperture or screen with commercial lamps to reduce the amount of radiation to the minimum required to visualize the vitamin Aon the column.)
Transfer the eluate to a suitable evaporation flask,and remove the Solvent Hexanecompletely under vacuum at a temperature not higher than 40 or with a stream of nitrogen at room temperature.Dissolve the residue in about 10mLof Solvent Hexane.
or with a stream of nitrogen at room temperature.Dissolve the residue in about 10mLof Solvent Hexane.
Second Column Chromatography—
Add the solvent hexane solution obtained as directed under First Column Chromatographyonto the Second Column.Rinse the evaporation flask with a total of 10mLof Solvent Hexanein small portions,adding each portion to the Second Columnand allowing it to flow through the column,and discard the effluent.When about 1mLof the hexane remains above the surface of the column,add 75mLof toluene,and elute with the aid of gentle suction (about 125mm of mercury),collecting the eluate.Evaporate the toluene under vacuum at a temperature not higher than 40 ,or with a stream of nitrogen at room temperature.
,or with a stream of nitrogen at room temperature.
Assay Preparation—
Dissolve the residue obtained as directed under Second Column Chromatographyin a small amount of Ethylene Dichloride,transfer to a 10-mLvolumetric flask,dilute with Ethylene Dichlorideto volume,and mix to obtain the Assay Preparation.
Color Development—
Into each of three suitable,matched colorimeter tubes of about 20-mm (inside)diameter,and designated tubes 1,2,and 3,respectively,pipet 1mLof the Assay Preparation.Into tube 1,pipet 1mLof the Standard Preparation;into tube 2,1mLof Ethylene Dichloride;and into tube 3,1mLof a mixture of equal volumes of acetic anhydride and Ethylene Dichloride.To each tube add quickly,and preferably from an automatic pipet,5.0mLof Color Reagent,and mix.After 45seconds,accurately timed,following the addition of the Color Reagent,determine the absorbances of the three solutions at 500nm,with a suitable spectrophotometer,using Ethylene Dichlorideas the blank.Similarly,45seconds after making the first reading on each solution,determine the absorbances of the solutions in tubes 2and 3at 550nm,in a similar manner.Designate the absorbances as A1500,A2500,A3500,A2550,and A3550,respectively,in which the superscript indicates the number of the tube and the subscript,the wavelength.
Calculation—
Calculate the quantity,in µg,of vitamin Din the portion of the sample taken by the formula:
(CS/C)(AU/AS),
in which CSis the concentration of vitamin D,in µg per mL,of the Standard Preparation;Cis the concentration of the sample (as g,capsules,tablets,etc.)in each mLof the final solution;AUhas the value of (A2500-A3500)-0.67(A2550-A3550)determined from the absorbances observed on the solution from the Assay Preparation;and AShas the value of A1500-A2500determined on the solutions from the Standard Preparation.
Biological Method
The biological assay of vitamin Dcomprises the recording and interpretation of observations on groups of rats maintained on specified dietary regimens throughout specified periods of their lives whereby the biological response to the preparation under assay is compared with the response to USP Vitamin D Capsules RS.
Preliminary Period—
Throughout the preliminary period in the life of a rat,which is not longer than 30days and extends from birth to the first day of the depletion period,maintain litters of rats under the immediate supervision of,or according to the directions of,the individual responsible for the assay.During the preliminary period,use a dietary regimen that provides for normal development but is limited in its content of vitamin D,so that when placed upon the Rachitogenic Dietin the depletion period the rats develop rickets.At the end of the preliminary period,reject any rat that weighs less than 44g or more than 60g,or that shows evidence of injury,disease,or anatomical abnormality.
Depletion Period—
Through the depletion period,which extends from the end of the preliminary period to the first day of the assay period,provide each rat ad libitum with the Rachitogenic Dietand water,and allow access to no other food or dietary supplement.
Rachitogenic Diet—
The Rachitogenic Dietconsists of a uniform mixture of the following ingredients in the proportions shown in the accompanying table.
Rachitogenic Diet
| Ingredient | Parts by weight |
| Whole yellow corn,ground | 76 |
| Wheat gluten,ground | 20 |
| Calcium carbonate | 3 |
| Sodium chloride | 1 |
When a chemical analysis of the entire ration shows a Ca:Pratio of less than 4:1or more than 5:1,the proportion of calcium carbonate may be varied to bring the adjusted ratio to a uniform level within this range.
Assigning Rats to Groups for Assay Period—
Consider a litter suitable for the assay period when individual rats in the litter show evidence of rickets such as enlarged joints and a distinctive wobbly,rachitic gait,provided that the depletion period is not less than 19or more than 25days.The presence of rickets may be established also from the width of the rachitic metaphysis upon X-ray examination or by applying the Line Test(described below)to a leg bone of one member of each litter.
Record the weight of each rat,and assign it to a group,in which each rat will be fed a specified dose of the Reference Standard or of an assay sample that is under examination for its vitamin Dpotency.For each assay sample provide one or more assay groups and not less than two standard groups.The two standard groups may be used for the concurrent assay of more than one assay sample.Within an interval not exceeding 30days,complete the assignment of rats to groups according to a design that divides litters among the groups,to achieve a complete balance.
For complete balance,whereby each litter is represented equally in every group,use 7or more litters containing at least as many depleted rats as there are groups.From a given litter,assign one rat,selected at random,to each group on the same day.If a litter contains twice as many rats as there are groups,assign a second series of rats similarly.The last one or two litters to be assigned may be allotted to groups so that at the start of the assay period the average body weight of any completed groups will not differ by more than 8g from that of any other group.
Assay Doses—
Select two dosage levels of the USP Cholecalciferol RS,spaced so that the ratio of the larger to the smaller dose is not less than 1.5or more than 2.5.Select one or two dosage levels based upon a single assumed potency for each sample.The dosage levels of the sample are equivalent to those of the standard or to a mid-level equal to the square root of the product of the two dosage levels of the standard.
Select dosage levels such that,when fed to rachitic rats,they are expected to produce degrees of calcification within the range specified under the test of data acceptability.Before feeding,the Reference Standard and/or sample may be diluted with cottonseed oil,provided that not more than 0.2mLis fed on any one day.Store the oil solutions in well-closed bottles,protected from light,at a temperature not exceeding 10 ,and use within 5weeks.
,and use within 5weeks.
Assign one group of rats to each dosage level of the standard and of the one or more samples.
Assay Period—
During the assay period,which extends from the end of the depletion period for a fixed interval of 7to 10days,cage each rat individually and provide it ad libitum with the Rachitogenic Dietand water.Supply a Rachitogenic Dietprepared from the same lots of ingredients to all rats.On the first and on the third (or fourth)day of the assay period,feed each rat one-half of its total assigned dose.
Throughout the assay period,maintain as uniform environmental conditions as possible for all rats,and exclude exposure to antirachitic radiations.At the end of a fixed period of 7to 10days,weigh and kill each rat.From those rats that do not weigh less at the end than at the start of the assay period and that have consumed each assigned dose within 24hours of the time it was fed,dissect out one or more leg bones for examination by the Line Test.
Line Test—
Remove the proximal end of a tibia or the distal end of a radius,and clean adhering tissue from it,in any one assay using the same bone from all animals.With a clean,sharp blade cut a median,longitudinal section through the juncture of the epiphysis and diaphysis at the same place on each bone.Rinse both sections in purified water,immerse immediately in silver nitrate solution (1in 50)for 1minute,and rinse again in purified water.Expose the cut surface of bone,in water,to daylight or another source of actinic light until the calcified areas develop a clearly defined stain without marked discoloration of the uncalcified areas.The staining procedure may be modified to differentiate more clearly between calcified and uncalcified areas.
Score the degree of calcification of the rachitic metaphysis in each rat,according to a scale that allows the average response to be plotted as a straight line against the logarithm of the dose.
Acceptability—
Observations are acceptable for use in calculation of the potency only from those groups in which two-thirds or more but not less than 7rats show calcification at least as great as the lowest level and not greater than the highest level.If the average score of the standard group on the high dosage level is not greater than the average score of the standard group on the low dosage level,discard the results,and repeat the assay.If an assay sample is represented solely by assay groups that are not acceptable for measuring vitamin Dpotency and in each of which the average score is less than the average score of the standard group on the low dosage level or more than the average score of the standard group on the high dosage level,its assayed content of vitamin Dis respectively less than that represented by the low dose or more than that represented by the high dose of the Reference Standard.
Calculation—
Tabulate the scores (y),listing each litter in a separate row with treatment groups in columns.Omit any groups that do not meet the test for Acceptability.Equalize the number of observations in the acceptable groups by disregarding the results on all litters not equally represented in the groups or by other suitable means (see Design and Analysis of Biological Assays á111ñ).Total the fscores for each of the treatment groups,where fis the number of litters,and designate each total as Twith subscripts 1and 2for the low and high dosage levels,respectively.Compute the slope bfrom the sums of T1,i.e.,ST1,and of T2,i.e.,ST2,for the standard and sample,provided the latter is represented at both dosage levels,from the equation:
b =(ST2–ST1)/ifh¢,
in which iis the logarithm of the ratio of the high dose to the low dose and is the same for each preparation,and h¢is the number of preparations represented by two dosage levels and included in the calculation of the value of b.
Compute the logarithm of the relative potency of each specimen under assay from the equation:
in which each mean score,bar(y)Ufor the assay sample and bar(y)Sfor the Reference Standard,is the average of the individual scores for an intermediate dosage level or of the two means for the high and the low dosage levels and where Tb=ST2–ST1and Tais as defined (see Design and Analysis of Biological Assays á111ñ).Convert each observedM¢to its antilogarithm to obtain the relative potency of the sample.Multiply the relative potency by the assumed potency of the assay oil in Units per g,adopted at the start of the assay,to obtain its assayed content of vitamin Din USP Units per g.