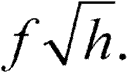Joint Assay of Several Preparations
Each monograph describes the assay of a single Unknown against the Standard.Although not provided explicitly,several different Unknowns are often included in the same assay and each is compared separately with the same responses to the Standard.This fact may warrant increasing the number of observations with the Standard.Given fobservations at each dosage level of each of hdifferent Unknowns,the number of observations at each dosage level of the Standard may be increased advantageously,if his large,to
This rule can be applied only approximately where litter differences or their equivalent must be segregated,and in any case is merely suggestive.
If all of several assays conducted concurrently meet the requirements for validity,and have linear log-dose response curves with the same slope band the same error variance s2about these lines,these two statistics may be considered as characteristic of the assay.Combining all of the evidence from the same assay into a single value of the assay slope results in a more stable and reliable estimate of bthan if each Unknown were analyzed independently.The degrees of freedom and reliability of the error variance s2can be increased similarly.Confidence intervals computed with these composite values for band s2are smaller on the average than if based upon only part of the relevant data.For the calculation or application of such assay estimates,see Equations 10,15,16,19,28,and 29.The potency estimated with a slope computed from a single Unknown and the Standard agrees within a fraction of the confidence interval with that computed from the combined slope for the entire assay.Since it is based upon more evidence,the latter is considered the better estimate.